Introduction:
Pediatric acute promyelocytic leukemia (APL) represents approximately 5%-10% of all acute myeloid leukemia (AML). While the prognosis of APL patients has dramatically improved with the use of all-trans retinoic acid and arsenic trioxide, cases of relapse still exist. Moreover, the molecular biological basis of pediatric APL remains unknown. In adult and pediatric AML, to predict prognosis, DNA methylation patterns have been proposed as biomarkers. In this study, we aimed to investigate the molecular biological basis of pediatric APL and validate biomarkers associated with prognosis using an integrated analysis, including DNA methylation analysis, targeted deep sequencing, and RNA sequencing, in patients enrolled in the Japanese AML-P05 study conducted by the Japanese Pediatric Leukemia/Lymphoma Study Group (JPLSG).
Methods:
Pediatric de novo APL patients (n = 38) who participated in the JPLSG AML-P05 trial from 2006 to 2009 were analyzed. Genome-wide DNA methylation analysis (n = 34), targeted deep sequencing (n = 35), RNA sequencing (n = 34), and motif analysis (n = 34) were performed. In the DNA methylation analysis, the Infinium MethylationEPIC v2.0 Kit (Illumina) was used.
Results:
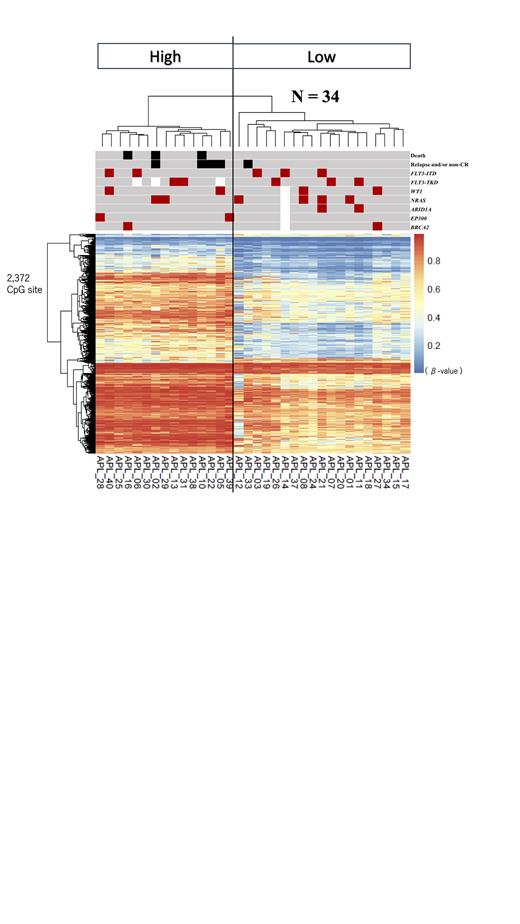
Based on the most variable 1,000 DNA methylation values between patients, unsupervised hierarchical clustering classified APL patients into four clusters (cluster 1, 2a, 2b, and 3). Clusters 1 and 2a (n = 15) demonstrated a significantly higher DNA methylation levels at 87 CpG sites out of 1,000 compared to clusters 2b and 3 (n = 19). A part of the 87 CpG sites locate on the genes, such as CPM, GRB10 and BAALC, which have been reported to be associated with APL prognosis. A significant DNA methylation difference was found between the “high” (cluster 1 and 2a) and “low” (cluster 2b and 3) methylation groups at 2,372 CpG sites (Figure). Patients in the high methylation group presented with significantly worse outcomes compared to those in the low methylation group (3-year overall survival: 80% vs. 100%, p = 0.043; 3-year event-free survival: 73.3% vs. 94.7%, p = 0.049).
Additionally, among the 2,372 CpG sites, motif analysis was performed for 783 CpG sites with an average methylation difference of 0.3 or higher. The binding domain of EBF1 was enriched among them. EBF1 is a critical transcriptional factor for B-cell differentiation. In the high methylation group, an aberrant B-cell differentiation was considered as a characteristic of APL.
Gene expression analysis between the high and low methylation groups revealed significant differences in expression of 600 genes with more than twofold changes. Gene ontology analysis of the 600 genes revealed that their genes were enriched in T-cell and B-cell linage differentiation.
In the targeted deep sequencing analysis, a total of 41 mutations in 21 genes in 25 patients were identified. The most frequent mutation was NRAS. Interestingly, previously unreported mutations in EP300 and BRCA2 were identified in APL, with EP300 mutations detected only in the high methylation group.
Conclusions:
DNA methylation analysis suggests that DNA methylation levels at specific CpG sites are effective biomarkers for the prognosis of pediatric APL. The presence of the EBF1 binding motif in the vicinity of these CpG sites suggests the involvement of EBF1 in prognosis.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal